Regulatory intelligence tool for seamless medical device classification
Determining your medical product classification can be a time-consuming and nerve-wracking process. The RAMS Product Classification service makes it simple to determine or verify the classification of your medical device or IVD product and focus on the regulatory pathway ahead.
Simplify medical device and IVD product classification

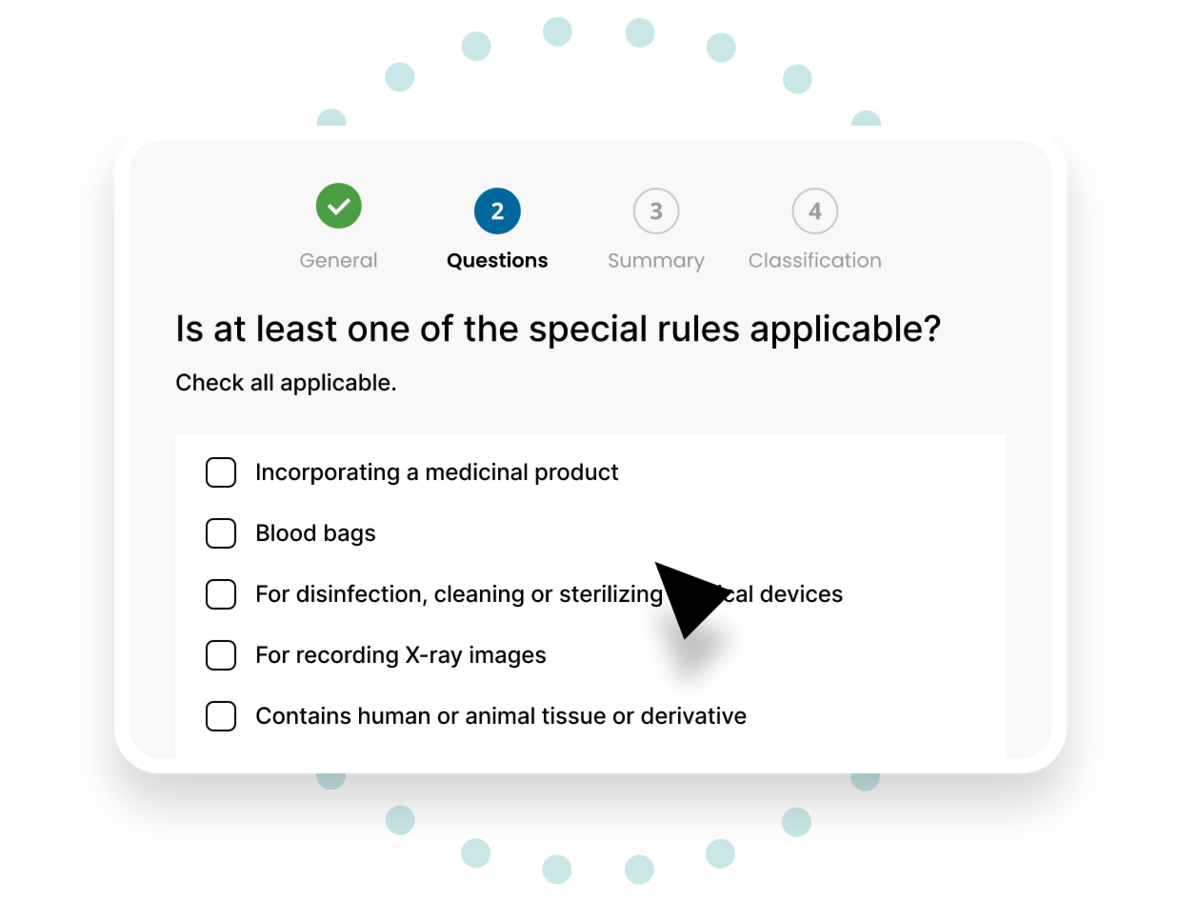
Quick guided results
Navigate a simple series of guided questions to get your result. Save time browsing classifications or searching for predicate devices. Get a quick answer that allows you to proceed with confidence.

Verify product classifications
Check your work if you previously located a product classification. Correct any false assumptions that could sabotage your route to market. Easy verification offers peace of mind for your team and investors.

Regulatory pathway strategy
Use your product classification to determine your regulatory pathway. Take advantage of complimentary and premium RAMS services like country data and Regulatory Intelligence. Get a fast start while avoiding mistakes to outpace any competitors.
Download MDR and IVDR reports
EU Regulatory Essentials report provides detailed information for various MDR and IVDR classifications. EU Classification Rationale indicates which regulatory rules apply to your device. The rationale can be incorporated into your Technical Documentation Report.
Join RAMS for smart medical device product classification
Create your complimentary account and see why RAMS is trusted by medical device companies all over the world.
Request information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.