September 5, 2024
By Beth Pugh, Annette Van Raamsdonk and Evangeline Loh
Returning from a long holiday and need to catch up on EU medical device and IVD regulatory developments? Read our update on European regulatory news over the last few months.
Amending legislation published to extend the transition of legacy IVDs and roll out EUDAMED
Regulation 2024/1860 was officially released to extend the transition time for legacy IVDs, roll out EUDAMED, and require data on disruption in supply as Article 10a Medical Devices Regulation (2017/745, MDR) and the In Vitro Diagnostics Devices Regulation (2017/746, IVDR). This was long awaited and formalized aspects that were discussed for over six months. Discussions related to Article 10a are still ongoing.
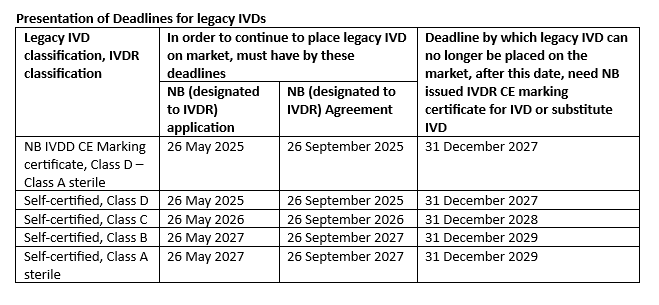
In the table below, we present the impending deadlines for legacy IVDs. One legacy element is that the IVD was placed on the market before the IVDR Date of Application (DoA), May 26, 2022.
The legacy IVD could continue to be placed on the market up until the date in the last column of the table, provided other conditions were fulfilled. This is based on the classification of the IVD to the IVDR, and the condition (Notified Body In Vitro Diagnostics Devices Directive 98/79/EC (IVDD) CE marking certificate or self-certified IVDD and up classified under the IVDR). Among the requirements is an IVDR application to a Notified Body and a signed agreement with the Notified Body for the legacy IVD or a substitute IVD by the dates specified in the table.

EC resources to support Regulation 2024/1860
The European Commission (EC) has posted resources to its website largely for legacy IVDs: press releases, fact sheets, Q&A documents and templates for the manufacturer’s Article 110 IVDR Declaration as well as the Notified Body template for the Notified Body notification letter (confirmation of manufacturer’s ability to continue to place the legacy IVD on the market).
The EC Q&A document is always helpful, as it shares practical information. And the documents and guidance related to Regulation 2024/1860 are consolidated in one site. Future updates will discuss the EUDAMED roll-out as well as reporting on disruption of medical devices or IVDs.
EC presents on the current regulations
In early July, the EC presented to international regulators on the “state of play”. This serves as a tremendous set of resources that summarize Regulation 2023/607, as well as Regulation 2024/1860. Both the legislative and non-legislative measures are included. The graphic representations and summary slides present the data in a readily understood manner. The session ends with a demonstration of the publicly accessible EUDAMED, and a reference to regulatory reliance (“information for international regulators who may or may not be practicing reliance on the EU regulatory framework”).
A recording of the presentation (two hours) is included.
EUDAMED vigilance module testing continues
The new version of EUDAMED (v 3.9.0) was deployed in the playground (not the production environment). Changes made to the various modules can be reviewed in the release notes that were published. Among the changes were some needed vigilance module updates for the Periodic Summary Report (PSR), PSR Periodic Analysis Update (PSRP), and Manufacturer’s Incident Report (MIR).
As with the previous release, We at Emergo by UL are again collaborating with a few competent authorities, a Notified Body and a manufacturer to test the system and offer feedback to the EC on issues encountered when submitting reports. There are several such teams collaborating to conduct validation-type testing of the vigilance module. The aim is to verify that the system is sufficiently functional and usable to perform the activities required by the regulations and thus confirm regulatory compliance.
Concluding remarks
Emergo Europe is a member of the European Association of Authorized Representatives (EAAR) and represents the EAAR in several EC meetings. As such we continue to monitor and report on the latest regulatory developments in the EU. We expect more on EUDAMED and the requirement to notify in the event of disruption of medical devices and IVDs in the upcoming month. Manufacturers will want to assess whether the new requirements (Article 10a) require revision of the Quality Management System.
The EC MDR and IVDR update also announced a targeted evaluation to assess the regulations. This public consultation is planned for the second week of September 2024.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.







