August 13, 2024
By Sarah Fitzgerald
We at Emergo by UL would like to point out that the U.S. Food and Drug Administration (FDA) has recently adapted certain searches and information to highlight regulatory guidance for specific medical device types. Medical device and IVD manufacturers who want to stay ahead of regulatory changes to avoid market access disruptions should take note.
Searching FDA guidance documents
The U.S. FDA has separated the "Search for FDA Guidance Documents" two sections by whether special controls are applicable or not:
- A search specific for guidance documents on Class II Special Controls Documents for specific device types
- A search for other guidance documents
The Class II Special Controls Documents, now a separate search (page, with a link from the main search page), provides guidance documents that mandate special controls.
The main search for guidance documents must still be utilized to provide standards that apply to many device types, such as biocompatibility, sterilization, software, cybersecurity, etc. It is important to note that this main search also includes Safety and Performance Based Pathway Guidance documents.
Searching FDA guidance documents for sutures, as an example
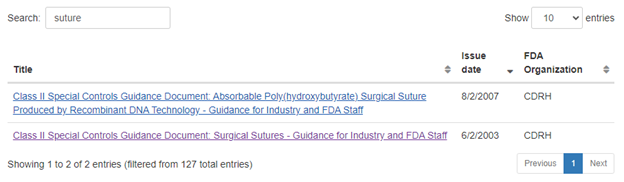
For example, if one searches for "suture" in the Class II Special Controls Document location, one will find two guidance documents:

These guidance documents indicate the applicable code of federal regulations (CFR) reference and/or the applicable product codes. For example, the 2003 guidance indicates the following:
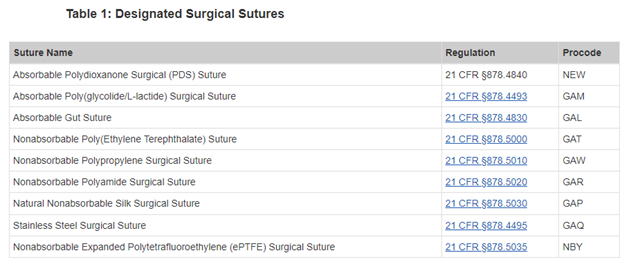
These documents then provide specific expectations for these medical devices.
If one searches for "suture" in the main guidance location, they will pull up one specific guidance document:

Note that a company also needs to consider other topics that may be relevant to a device and should search for FDA guidance on those topics. So additional searches would need to be conducted to find these broadly applicable guidance documents.
Searching by product classification
Second, the FDA has updated its product classification results for specific product codes. The initial search is the same, but when a certain product code is selected, the relevant device-specific guidance documents will be included in addition to the recognized consensus standards that were previously indicated. Using the same example, if one searches for "suture" from the product classification search, 31 results are returned. If one is chosen, the specific information is provided, as in the example below:
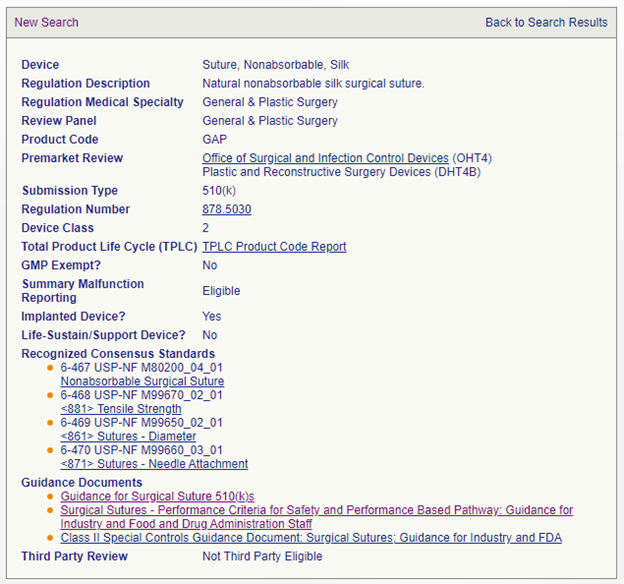
Note that the device-specific guidance documents from both the special controls and the general guidance document searches are now included. However, broadly applicable guidance documents (such as biocompatibility) are not included, in the same manner that broadly applicable standards are not (and have not been) included in such a search.
Additional notes
We at Emergo by UL would like to point out that during this update, not all browsers were appropriately returning results. Although this appears to have been resolved for common browsers for the time being, if this is an issue, the browser cache may need to be cleared or a different browser may need to be utilized.
Concluding remarks
When bringing a device to the U.S. market, it is critical to understand the FDA's current thinking related to this device, as communicated through guidance documents. This update should make it easier for a company to find guidance documents specific to a device type when searching through the product classification search.
However, when searching guidance documents, companies must be aware that they should search both the Class II Special Controls Documents (where relevant) and the general guidance document search to confirm that they are not missing relevant FDA guidance. Additionally, you should continue to use the general guidance document search for topics broadly applicable to various medical devices.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.



