September 19, 2024
By Karen Hill and Evangeline Loh
On September 18, Robert Reid, Ph.D. Deputy Director, Innovative Devices at the Medicines and Healthcare products Regulatory Agency (MHRA), updated the RAPS audience on the current status of the UK legislation. To our knowledge, this is one of the most recent updates from the MHRA.
The first half of 2024
In January 2024, the MHRA shared a roadmap for 2024-2025. The roadmap proposed regulatory reforms to the UK MDR 2002 based on previous reports and consultations. Three core legislations (statutory instruments (SI)) are envisioned: post-market surveillance (PMS), pre-market/core legislation, and future enhancements. Notification of the draft PMS SI was published on the WHO website at the end of July 2023, and it was expected to enter into force in June 2024, which has not yet happened.
Around the same time as the election of the new UK government, in May 2024, Dr. Reid assumed his new position at the MHRA. Under the new labor government, Baroness Merron was appointed as the Parliamentary Under-Secretary of State at the Department of Health and Social Care in July, all of which have impacted the legislative process.
Refreshed roadmap
Given the dissolution of Parliament at the end of May and the election in July, the proposed timeline was considered ambitious and was extended. Consequently, the MHRA has been reassessing, as well as focusing on the most important tenets, patient safety and patient access.
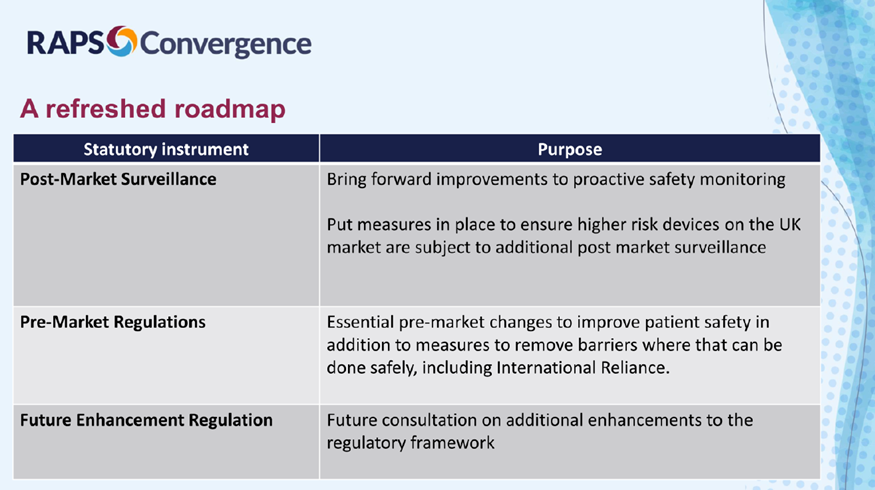
Dr. Reid presented the following slide at RAPS titled “A refreshed roadmap” and explained that the three core SIs would still generally remain the same.

PMS SI
The PMS SI is expected to be presented in the next two months before Parliament. The draft SI shared in 2023 will be the version presented to Parliament. The more stringent PMS requirements aim to improve patient safety and provide clarity on PMS requirements, as well as provide a more harmonized approach and improve identification and analysis of medical device incidents. Another benefit of improving the PMS process is that it would subject medical devices to the same rigorous PMS process, including the devices that in the future would take the proposed reliance routes.
Pre-market statutory instrument
The essential changes to the core legislation are anticipated to be released in 2025. These would include the new requirements for UDI, implant cards, claims made and changes in classification. Dr. Reid reminded the audience that the legislative changes would be guided by the government’s response to the consultation on the future regulation of medical devices in the UK published in 2022.
In addition, the legislation will be updated to include the international reliance routes announced by the MHRA in May 2024. The MHRA has been testing the reliance route with manufacturers of different devices and classes to see how it would function.
Stephen Lee, Director of Diagnostics & Digital Regulation, ABHI, was also a presenter on the RAPS panel. He had attended the first two days of the International Medical Device Regulators Forum (IMDRF) meeting and acknowledged the global attention on reliance. He complimented the MHRA on their work towards establishing a regulatory process based on international recognition and reliance.
Future enhancement SI
The MHRA still intends to conduct a third SI, on which they will work with stakeholders in open discussions.
Concluding remarks
While the initial roadmap has been delayed, the MHRA will still proceed with three SIs. In addition, one of the most significant updates is the inclusion of the reliance system in the pre-market stage.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.







