January 29, 2025
By Michael Orduz
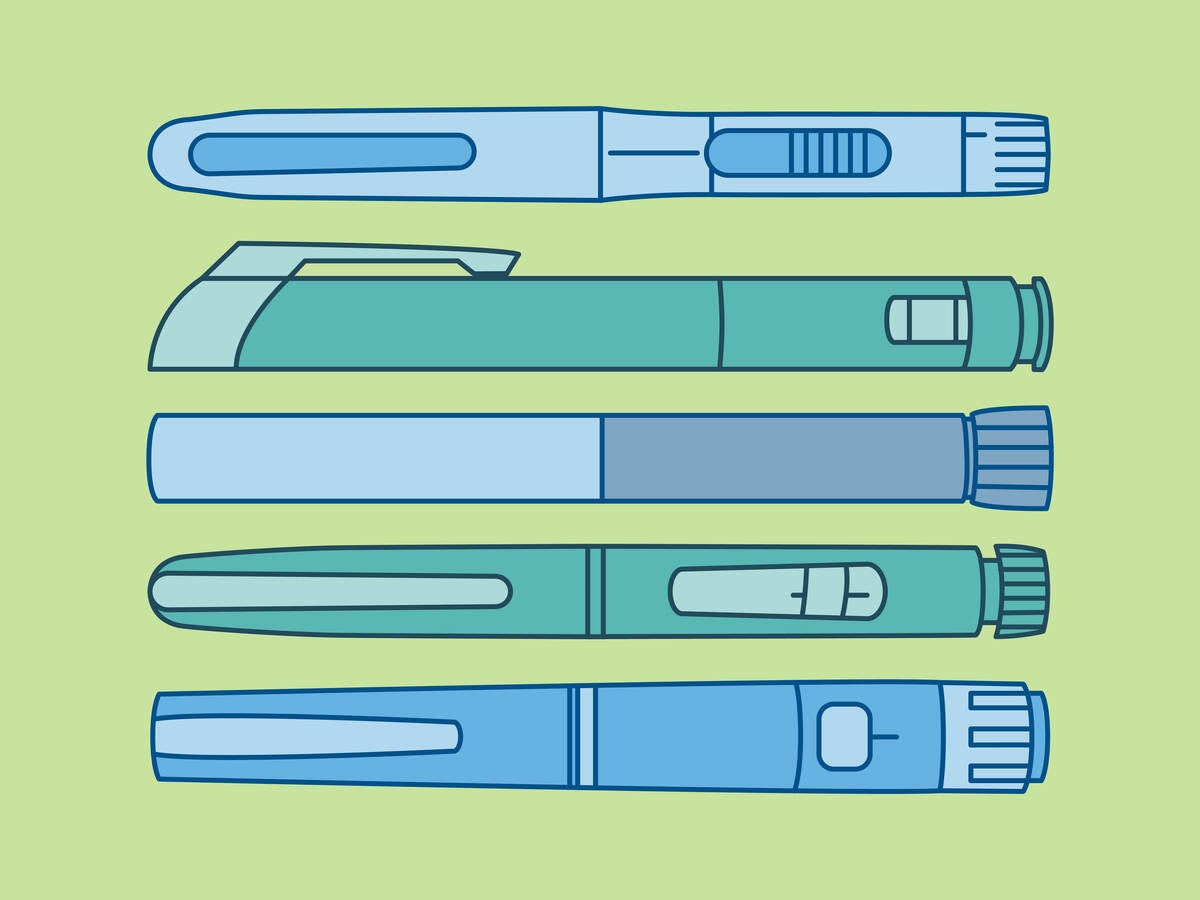
Understanding pen-injector use errors and their implications
The proliferation of pen-injectors used by laypeople for self-administration of medication in their homes has caused regulators, such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), to task manufacturers with providing sufficient evidence to demonstrate that all users, especially laypeople, can safely and effectively administer an accurate dose. Much of this evidence is comprised of manufacturers anticipating use errors through preliminary analyses and, to the extent possible, “designing out” these potential errors to create a more intuitive, safe, and effective product design.
Defining use errors and their role in risk mitigation
Per the FDA, a use error is defined as a: “User action or lack of action that was different from that expected by the manufacturer and caused a result that (1) was different from the result expected by the user and (2) was not caused solely by device failure and (3) did or could result in harm.”
Strategies to prevent pen-injector use errors
And, how should we go about mitigating these use errors?
-
Mitigate through design. The preferred and most effective approach to reducing use-related harm focuses on designing the product’s user interface such that use errors are eliminated.
Example: Pen-injector can only deliver medication if dose counter is turned to a value above zero (“0”)
-
Implement protective measures. A secondary approach to reducing use-related harm is introducing design characteristics that do not eliminate the potential use error but make them highly improbable.
Example: Pen-injector with cap and intuitive gripping area
-
Information for safety. Another valuable, yet less effective, risk control measure is to revise the product’s labeling (e.g., instructions for use) and associated training to mitigate use errors.
Example: Clear pen-injector packaging
Analyzing common errors and their mitigation through design
Below we discuss some of the top use errors observed during our experience conducting hundreds of pen-injector usability tests, as well as relevant potential root causes and design recommendations.
-
User does not inspect (e.g., for damage, tampering) the pen-injector and/or the associated packaging / medication. This use error is commonly caused by insufficient use of graphics in the pen-injector’s IFU. Specifically, some steps, such as inspecting the device and/or packaging, are not depicted as IFU graphics. Consequently, users might overlook certain steps in their interactions with the pen-injector. To reduce the likelihood that this use error continues to occur, manufacturers should consider increasingly illustrating critical steps (i.e., ensuring all critical steps have an associated illustration to catch the user’s attention).
-
User does not prime the pen-injector’s needle. Pen-injectors are sometimes not primed (i.e., small dose expelled to remove air bubbles from the needle) because users perceive the pen-injector as ready to inject and, therefore, do not intuit the need to prime the pen-injector. This use error can be combated by adding an indication on the pen-injector itself of a primed versus unprimed pen-injector. Manufacturers might also consider including measures to prevent users from injecting before priming (e.g., cannot push button until user has turned dose counter to a specific dosage).
-
User sets the pen-injector’s dose incorrectly. This use error is often attributed to inconspicuous decimal point values. As such, manufacturers using dose dialer’s with decimals are urged to, for example, increase the prominence of the tenths place number to aid in catching the user’s attention. Additionally, a manufacturer could only include haptic feedback on the dose dialer’s tenths values and remove haptic feedback from the hundredths values.
-
User removes pen-injector needle before prescribed dose has been delivered. Some pen-injectors are designed with an inconspicuous indicator of injection completion. Meaning that the dose pointer does not clearly line up with the “0” on the dose counter and, as such, users might remove the pen-injector prematurely. Furthermore, a pen-injector’s IFU step might only depict pressing the dose button (i.e., does not clearly specify to press and hold the dose button). To mitigate this use error, manufacturers can leverage multiple feedback modalities. For example, they could leverage audible (e.g., clicks) and/or haptic feedback to indicate when the injection is ongoing versus completed. They could also clarify injection duration by stating in the relevant IFU step that users should start counting to 10 once they have fully pressed the “thumb button” down, rather than when they start pressing the button.
-
User does not dispose of the pen-injector’s needle into a sharps container. Users overlooking to discard the needle appropriately usually points to the associated IFU step providing insufficient detail (e.g., does not include acceptable disposal location; sharps container graphic). Accordingly, manufacturers can elect to improve the disposal step by adding detailed graphics depicting the acceptable disposal location / receptacle (e.g., clearly labeled sharps container with country-appropriate color).
Using evaluations to predict and reduce pen-injector use errors
The majority of use errors can be predicted by performing thorough formative evaluations and by learning lessons from similar combination products. Fortunately, many use errors can also be designed out of a product. By thoroughly identifying the root cause(s) of use errors observed during usability testing, you empower your product designers to refine the user interface with precision. Their expertise enables them to turn these insights into intuitive solutions, ultimately minimizing the potential for pen-injector use errors and setting up users for a positive experience with your product.
Leveraging human factors insights to mitigate use errors
Contact our team to learn more about pen-injector and other combination product use errors and how to mitigate them. Or, sign up for a complimentary account with OPUS, our team’s software platform that provides HFE training, as well as a Root Cause Analysis Wizard and a Design Recommendation Library.
Michael Orduz is a Senior Human Factors Specialist at Emergo by UL.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.