May 3, 2024
By Lisanne de Vogel
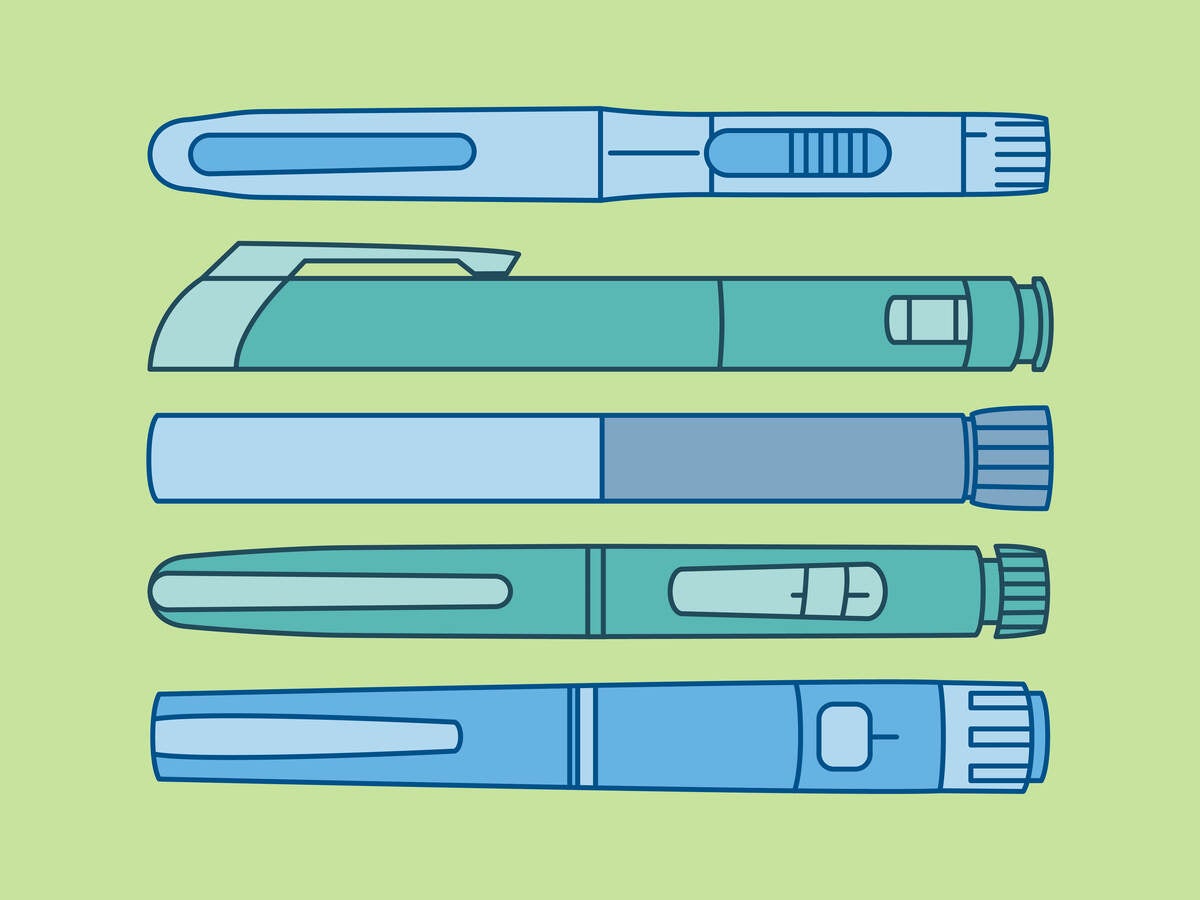
With the increasing use of drug-device combination products such as pen injectors, pre-filled syringes and inhalers, there is a growing chance for potential medication mix-ups. People from the same household might use similar products, for example when multiple family members have diabetes. Or people with asthma might have different dose sizes of the same inhaler available at home. How can manufacturers prevent potential medication mix-ups from occurring? And are there any regulatory requirements to consider? This article discusses some key considerations for ensuring your drug-device combination product is sufficiently differentiable from others on the market to avoid people taking the wrong medication.
Understanding medication mix-ups
Medication mix-ups, or “medication errors,” are defined by the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) as “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer.” A key term in this definition is “preventable event.” When is an error considered “preventable” versus when is something “off-label use?” FDA’s Center for Drug Evaluation and Research (CDER) considers a preventable event as “events that are due to errors that could be avoided”. It provides the example of receiving an incorrect drug due to similar-looking labeling. This implies a responsibility for the manufacturer to ensure their drug-device combination product is sufficiently designed to prevent medication mix-ups are prevented.
Regulatory imperatives for product differentiation
When bringing a combination product to market, most manufacturers nowadays are familiar with the FDA’s and IEC 62366’s guidance on conducting a Human Factors (HF) validation or summative study and submitting an HFE report or usability engineering file to regulators. But what if you are planning to market a new dose strength of your existing combination product, or market multiple dose strengths at the same time? Does that mean full HF validation testing that is focused on handling is required? And should you evaluate if your users can differentiate between multiple dose sizes or similar products on the markets?
Regulators do not officially require manufacturers to conduct differentiation studies. However, that does not mean there is no focus on risks related to medication errors. For example, within the US FDA’s CDER the Division of Medication Error Prevention and Analysis (DMEPA) reviews and advises manufacturers in which ways to minimize medication errors. They evaluate the product name and also labeling, instructions for use, the physical product’s design, and HF data.
Moving over to the EU, EMA has published some “good practice” guidance documents informing manufacturers about reporting, evaluating, and preventing medication errors. Specifically, the guide on risk minimalization and prevention of medication errors states that “The potential for medication errors should be considered at all stages of the product life-cycle but particularly during product development.” They recommend considering different factors when designing a product, for example using color, increased font size, and warnings.
Exploring different types of combination products
Not all drug-device combination products might fall into the same risk category. Both FDA and EMA seem to place a specific focus on high-risk combination products. For such products, taking the wrong medication or dose can have serious clinical consequences that are potentially life-threatening. Insulin or insulin-type products are a good example. There are many injection devices on the market treating conditions like diabetes, and the different variations can have very different effects on patients. If a patient mistakes their long-acting insulin for a short-acting insulin, the impact can be severe.
EMA has even published a specific addendum to their guide for medication errors associated with “high-strength and fixed-combination insulin products.” This guide specifically encourages manufacturers of these insulin products to apply any color differentiation on the labeling but also to the device itself. Additionally, the addendum emphasizes taking designing for visually impaired or color-blind users into account, for example by also applying a tactile structure to the device.
Key considerations for effective product differentiation
As a drug-device combination product manufacturer, there are several key considerations to take into account when developing a new product and/or dose variant:
- Familiarize with the regulatory guidance documents available. Making an effort to understand regulators’ current perspectives and considerations can help shape your development process.
- Investigate known problems/adverse events with a focus on medication errors. What information is currently available about medication errors with your existing product(s)?
- Investigate the current market landscape in terms of packaging design, device design, product name, color schemes, etc. Apply recognized HF design principles to your product and its packaging, for example, follow the recommendations outlined in ANSI/AAMI HE75:2009.
- Investigate relevant comparator products. What is currently out there that might be similar or related to your product? What does the packaging and product design look like?
- Implement HFE early in your product design process. Applying HFE principles ensures your product stands out in terms of name, packaging color, packaging design, and product design. For example, use multiple visual cues rather than using only color as a differentiating factor, taking people with visual impairments or color blindness into account
Conducting differentiation studies: best practices
When you are ready to put your design to the test, you can conduct a standalone differentiation study or include differentiation tasks in your “regular” HF study. A good starting point to shape your methodology is to select relevant comparator products. Then, you can accurately evaluate how your product differs from others on the market. Which other medications out there are often prescribed to your intended users? In case your product is already on the market, or you are planning to market multiple dose strengths at the same time, remember to also include different dose variants as comparator products. For example, users might switch to a higher dose and therefore might have access to multiple variants at some point.
Our team has vast amounts of experience conducting differentiation studies of combination products, including our design team, whose expertise can support efforts to make your product sufficiently different from others. For support with your combination product or medical device development, contact our global team. Or, sign up for a complimentary account with our human factors software, OPUS, Emergo by UL’s software platform that provides human factors engineering (HFE) training, tools, and templates.
Lisanne de Vogel is a Senior Human Factors Specialist at Emergo by UL.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.