March 12, 2025
By Amelia Boldrick and Elizabeth Pugh
The technical document Terminologies for Categorized Adverse Event Reporting (AER): Terms, Terminology and Codes has been updated by The International Medical Device Regulators Forum (IMDRF) Adverse Event Terminology working group (AET WG).
The current update consists of 36 newly added terms, changes to 56 existing terms and the retirement of eight terms. Release notes that provide a high-level overview of specific code updates in the 2025 publication cycle are available from the IMDRF website.
Harmonization of categorization of adverse events
IMDRF AET is intended to harmonize the categorization of adverse events across global markets to improve signal detection for both regulators and industry.
The terminology is maintained by the AET WG. New terminology, codes and definitions, as well as the retirement of or changes to existing ones, can be requested by IMDRF stakeholders on an annual basis. IMDRF/AE WG/N44FINAL:2020, Maintenance of IMDRF AET governs the process for submitting change requests. It allows competent authorities or stakeholder organizations (defined in guidance as an organization that has any direct or indirect interest in medical devices or in vitro diagnostics such as industry associations, professional associations, professional medical associations, patient associations, etc.) to submit requests to AET WG.
Change Requests can be submitted annually for review from September 1 of the current year to September 1 of the following year. After the window closes, the AET WG reviews submissions and makes recommendations to the IMDRF Management Committee for final decision. Accepted changes are published in March, along with a Change Log with the details of each request and outcome rationale.
We at Emergo by UL previously reported on the draft guidance, IMDRF/AET WG/N86 DRAFT: 2024 Considerations for the selection of IMDRF AET, for which the consultation period recently closed. It clarifies that the coding was developed to meet the needs of regulators, noting that manufacturers may need more specific terms for coding their product post-market surveillance data. The draft guidance suggests that a manufacturer can develop additional child terms/codes for their own use. When used in a report the IMDRF code would be used for reporting not the additional “child” term created by the manufacturer.
2025 terminology updates
The 2025 Change Log is available for review. It outlines the individual outcomes of all 140 requests submitted between five national regulators, two industry associations, and three manufacturers.
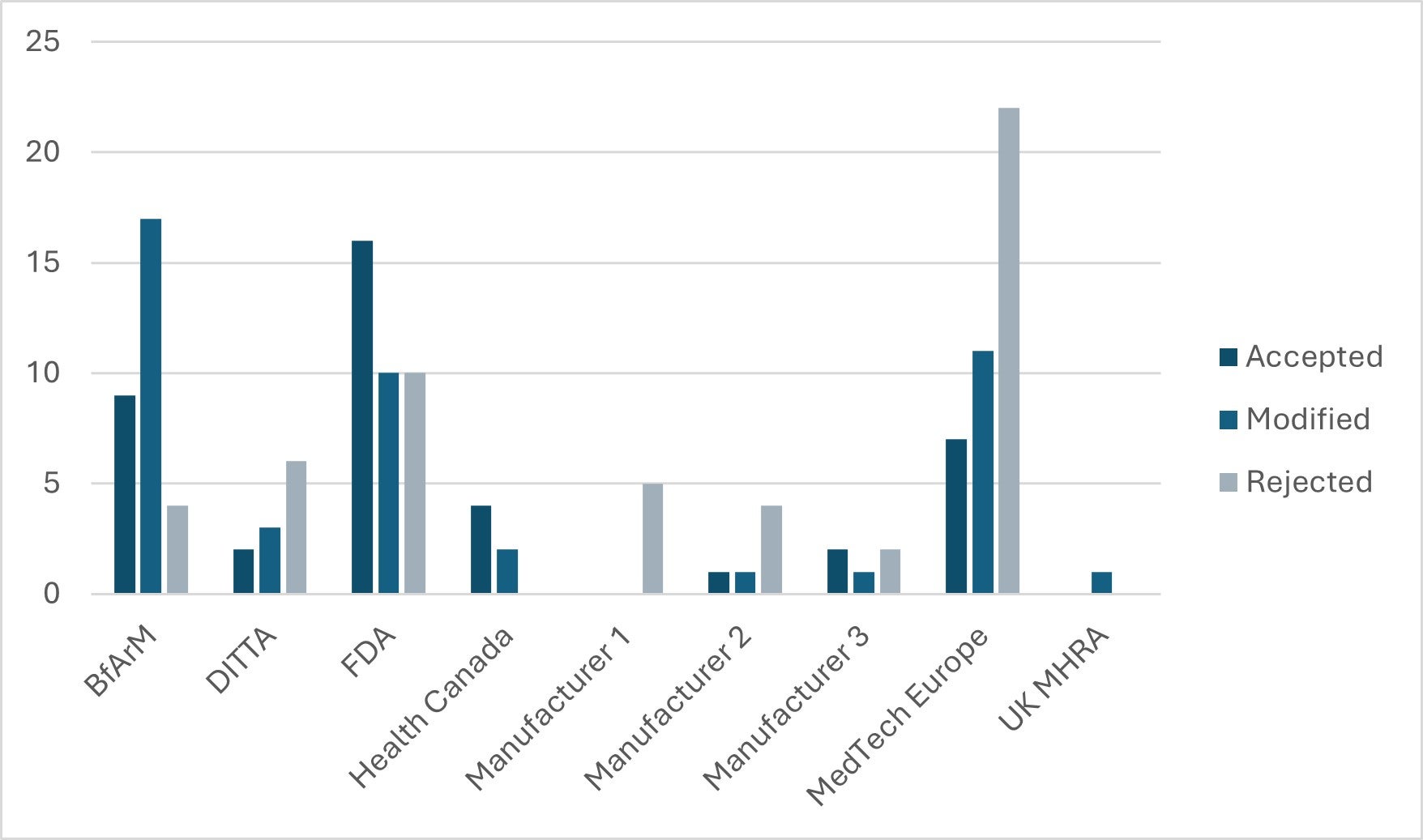
Figure 1: Change Request Outcomes by Submitter
Although a narrow majority of the change requests submitted last year came from regulators, a disproportionately high number of rejections were received for requests from industry.
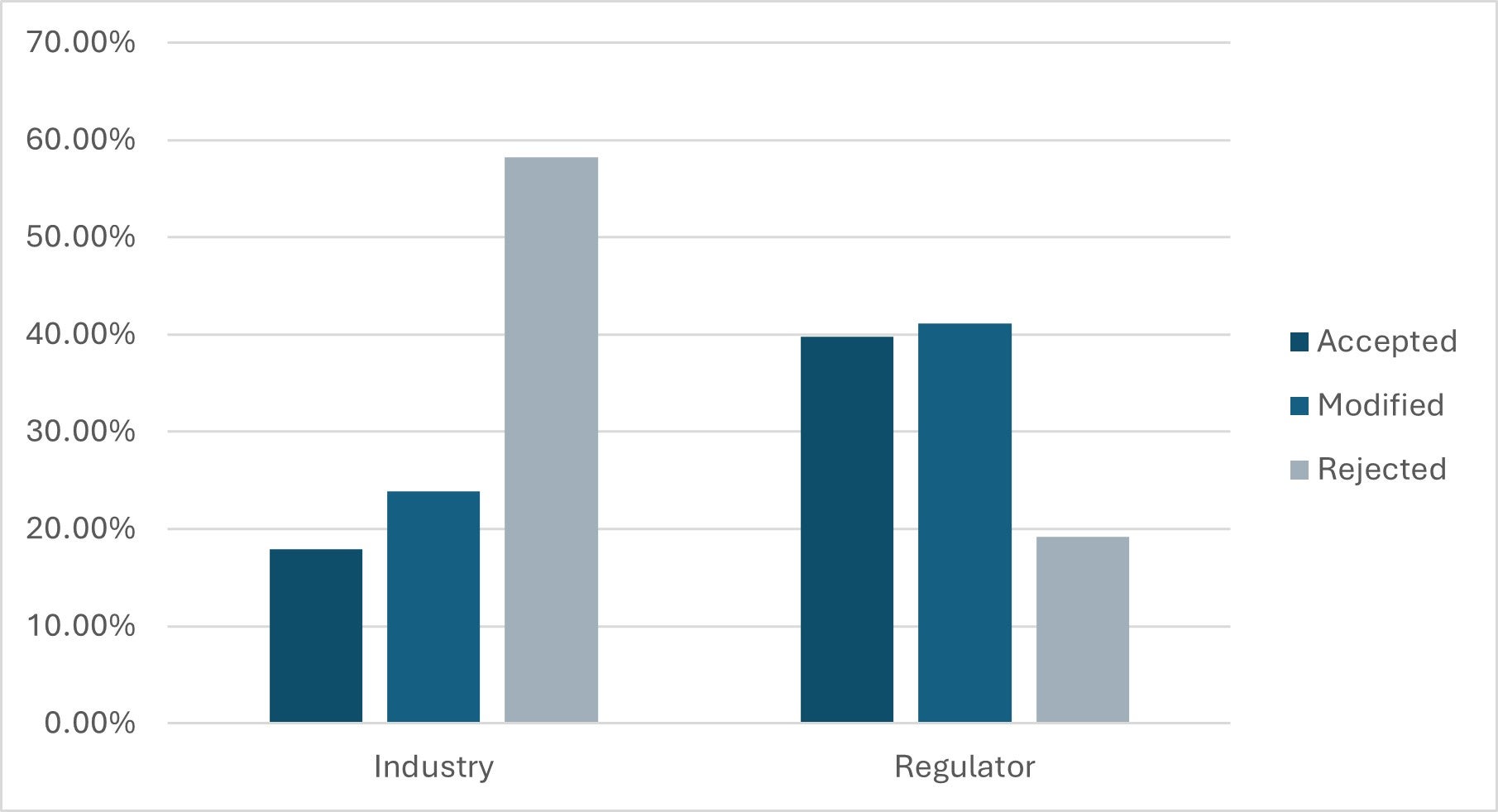
Figure 2: Change Request outcomes as a percentage of total submitted by stakeholder role
Notable among the changes accepted are seven new Annex B terms (Cause Investigation - Type of Investigation). Annexes B, C, and D, representing the manufacturer investigation and outcome, contain comparatively fewer terms than the annexes pertaining to device problems, components, and health impact. The 2025 update brings the total number of terms for manufacturer investigation types from 24 to 31.
Annex B is still the smallest in terms of the total options available. However, in terms of new additions, it was second only to the largest annex; Annex E (Health Effects - Clinical Signs and Symptoms or Conditions), which picked up 19 new terms this year.
Administrative updates
Those who refer frequently to the AET will benefit from two administrative updates that improve the form and function of the coding resources provided by the IMDRF.
Annexes A – G can now be accessed in a single spreadsheet file, whereas before it was necessary to download each annex in a separate file.
Those who prefer to view the codes, terms and definitions in their web browser will be pleased to know that it is also now possible to copy and paste them directly from the annex-specific pages.
Concluding remarks
IMDRF AET is an important tool for identifying trends and safety signals. Manufacturers should use IMDRF AET in risk management files and map in-house complaint codes to IMDRF AET to detect potential product problems as quickly as possible.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.