January 30, 2025
By Sade Sobande
The International Medical Device Regulators Forum (IMDRF) released two key documents related to medical device software: Good machine learning practice for medical device development: Guiding principles (IMDRF/AIML WG/N88 FINAL:2025); and Characterization Considerations for Medical Device Software and Software-Specific Risk (IMDRF/SaMD WG/N81 FINAL:2025). These documents are aimed at improving global harmonization and guiding medical device manufacturers in navigating compliance for software-based and AI-driven technologies.
Note: The IMDRF guidance documents are referred to by the N numbers.
Good machine learning practice for medical device development: Guiding principles
The Good Machine Learning Practice (GMLP) principles outlined in the IMDRF document are aligned, except for minor refinements in wording, with those jointly identified by the U.S. Food and Drug Administration (FDA), Health Canada, and the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA). A key difference is the scope of collaboration and intended audience, which makes it more relevant to global manufacturers.
Key takeaways
The principles intended to assure the development of safer, more effective and high-quality AI/ML-enabled medical devices. Three fundamental tenets may be derived from the principles:
- AI models must be transparent and explainable to enhance regulatory trust;
- Robust data management practices are critical to support fairness and minimize bias;
- Risk management is crucial, and must take a total product lifecycle approach.
The 10 principles of good GMLP software are summarized below (Figure 1):
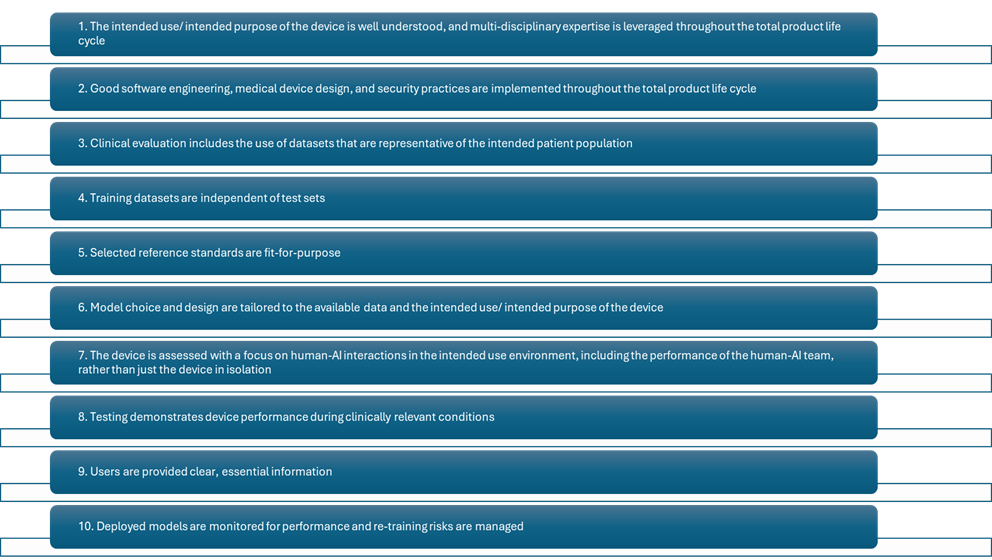
Figure 1: Snapshot of the 10 GMLP Guiding Principles
The release of the N88 document marks a major step towards the harmonization of ML principles for medical devices. Manufacturers of ML-enabled devices that integrate GMLP into their product lifecycle will be well placed to benefit from improved compliance, reduced regulatory hurdles, enhanced product reliability and customer trust.
Characterization considerations for medical device software and software-specific risk
Building on the risk categorization framework established in IMDRF/SaMD WG/N12 FINAL:2014, this new N81 document has been advanced to take into account the diverse landscape of software and its varied interpretation across different territories. There is alignment in the terminology used in the EU MDR / IVDR with the use of the term, ‘medical device software’ (MDSW). This expands the scope of the N81 document to include embedded software (software in a medical device, SiMD) which is a much welcome addition, although it must be emphasized that the N81 document is intended to complement not replace the N12 framework. The document provides valuable guidance on how to effectively characterize MDSW and assess associated software-specific risks.
Key takeaways
- MDSW characterization requires a clear intended purpose and device description.
- A well-defined intended purpose statement supports clarity and aids in risk assessment. The key elements outlined repeat those of the MHRA document, Crafting an intended purpose in the context of Software as a Medical Device (SaMD);
- MDSW characterization should consider the medical problem, context of use, functionality and change management, including degree of learning and autonomy;
- The risk characterization strategy must consider both direct and indirect harms. This aligns with established risk management principles such as those in ISO 14971. In particular, the document highlights the importance of understanding the contribution of information-related hazardous situations and consequently how software-specific harms can lead to risks unique to a given intended purpose;
- Taking a harmonized approach to software characterization is important for consistent and fair regulation. Stakeholders are encouraged to integrate and leverage where possible the concepts and principles outlined in the document in order to promote clear, consistent and accurate characterizations of MDSW.
Medical device software is changing fast
Medical Device Software, particularly AI/ML-enabled devices is evolving rapidly. For us in the medical device industry, international collaboration and global harmonization are essential to reducing regulatory fragmentation, ensuring consistency in MDSW regulation, and streamlining market entry. These two IMDRF documents are welcome additions to the goal of fostering international harmonization and will play a crucial role in the marketing strategy of manufacturers seeking multi-market access for such devices.
The IMDRF working groups continue to make progress, and we at Emergo by UL will continue to share the developments.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.






