April 1, 2024
By DaYeon Choi
In our last article inspired by KIMES 2024, we at Emergo by UL shared the synopsis of our seminar on the European (EU) amending legislation, Regulation 2023/607.
This series featured the following articles:
- Primer on South Korea medical device regulatory system;
- Insight about TPA and or MFDS observations during K-GMP audits;
- Acceptance of MFDS of MDSAP audit results in lieu of on-site K-GMP audits; and,
- KIMES 2024 update on US FDA eSTAR.
To continue our KIMES 2024 coverage, we discuss our presentation on European Regulation 2023/607.
Key changes to the regulations under the amendments laid out in Regulation 2023/607
Regulation 2023/607 entered into force on March 20, 2023. We discussed the key changes per the Regulation 2023/607 and the amendments to the EU Medical Devices Regulation (MDR, 2017/745) and In Vitro Diagnostic Devices Regulation (IVDR, 2017/746) and the impact on legacy medical devices and IVDs, respectively. The Regulation amends Article 120 and Article 122 of the MDR, as well as Article 110(4) and Article 112 of the IVDR.
So what are the key changes to Regulation 2023/607?
- The “Sell-off” provisions in the MDR and IVDR are deleted. This implies that devices placed on the market before the date of application (DoA) of the MDR (May 26, 2021) and the IVDR (May 26, 2022), following the Medical Devices Directive (MDD, 93/42/EEC), Active Implantable Medical Devices Directive (AIMDD, 90/385/EEC), and In Vitro Diagnostic Medical Device Directive (IVDD, 98/79/EC) during the new transitional provisions, may continue to be made available on the market or put into service without any time limits.
- MDD and AIMDD certificates are extended under certain conditions.
- Class I self-certified medical devices, up-classified per the MDR, also benefit under certain conditions.
Using Regulation 2023/607 on devices certified under MDD/AIMDD to extend the transitional period
We focused on Regulation 2023/607 amendments to the MDR, the new transitional provisions depend on the risk class of the device. It is important to emphasize that the risk class of the device is based on Annex VIII Classification Rules of the MDR, not Annex IX of the MDD.
‘Certificates issued by notified bodies in accordance with Directives 90/385/EEC and 93/42/EEC from 25 May 2017 that were still valid on 26 May 2021 and that have not been withdrawn afterward may take the full advantage of the Regulation 2023/607 amendments regarding the extension of transitional period if the following conditions are fulfilled:
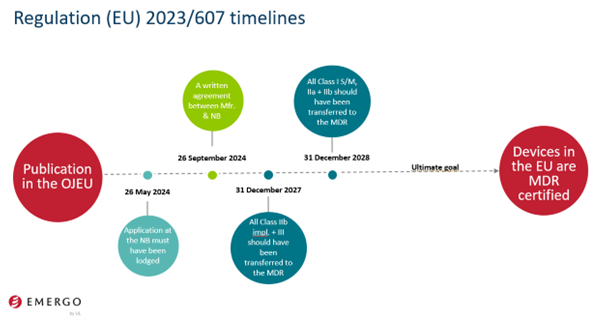
- A formal application for conformity assessment of devices with a quality management system per MDR has been submitted to a notified body before May 26, 2024, and the manufacturer and the notified body have signed a written agreement for the devices covered by the certificate before September 26, 2024.
- A competent authority of a Member State has granted a derogation from the applicable conformity assessment procedure under Article 59(1) of the MDR or has required the manufacturer to carry out the applicable conformity assessment procedure per Article 97(1) of the MDR before the publication of Regulation 2023/607.
This typically means the MDD and AIMDD certificates were still valid between March 20, 2023 to May 26, 2024, and there are no significant changes in design or intended purpose (ongoing compliance to MDR Article 120).
What is the additional time?
Suppose one of the above conditions is met. In that case, the devices under the MDD/AIMDD certificates may be placed on the market or put into service until the following dates, provided continual compliance with Article 120:
- December 31, 2027, for all class III devices, and class IIb implantable devices, except sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips and connectors.
- December 31, 2028, for class IIb devices other than those covered by point (a) of this paragraph, for class IIa devices, and for class I devices placed on the market in sterile condition or having a measuring function.
- Class III custom-made implantable devices may be placed on the market or put into service until May 26, 2026 without a certificate issued by a notified body.
Note again, MDD Class I self-certified legacy devices, up-classified per the MDR, are also included provided the conditions are met.
We shared a timeline slide for visual awareness related to Regulation 2023/607.

Concluding remarks
The May 26, 2024 deadline is rapidly approaching. For manufacturers of legacy medical devices to continue to place the legacy devices on the market after this date, there must be an application with a notified body designated for the MDR. By September 26, 2024, manufacturers of legacy devices must have a signed agreement with the notified body.
Because of the removal of the sell-off provision, it would appear that [LE1] manufacturers can place these legacy devices on the market before the May deadline, and provided these devices are in the supply chain, the distributors can sell them off.
KIMES 2024 was a fantastic conference and we’re already anticipating next year!
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.






