April 10, 2025
By Evan Ruby and Lori White
The Australian Therapeutic Goods Administration (TGA) has announced that its Unique Device Identification (UDI) regulatory framework is now in effect following the approval of the Therapeutic Goods Legislation Amendment (Australian Unique Device Identification Database and Other Measures) Regulations 2025. Additionally, the TGA has published new guidance and updated its website to reflect the commencement of the UDI regulatory framework, available via the TGA’s Unique Device Identification Hub.
UDI requirements
The Essential Principles have been amended to set out requirements related to UDI, including:
- Obtaining a UDI-Device Identifier (UDI-DI)
- Formatting the UDI-Production Identifier (UDI-PI)
- Including UDI in labeling, packaging, and Patient Implant Cards
- Direct marking of devices with the UDI-DI
- Submitting data in the Australian UDI database (AusUDID)
The TGA has provided further details on UDI labeling requirements on its website. The TGA’s new guidance, Complying with the Unique Device Identification Requirements for Medical Devices, outlines the requirements for obtaining and applying a UDI, as well as submitting and maintaining UDI data in the AusUDID.
All devices are subject to these UDI requirements, except for Class I and Class I (measuring) devices. Class 1 in vitro diagnostic devices (IVDs) are also exempt, unless they are considered instruments or software. Manufacturers may choose to assign and apply a UDI to exempt devices; if this is done, the TGA recommends meeting all UDI requirements to avoid confusion for end-users.
Implementation of UDI requirements and compliance start dates
Compliance with the above-mentioned UDI requirements will be implemented in stages. The “compliance start date” will depend on the device class, whether the device is supported by the EU Medical Device Directive (MDD) or In Vitro Diagnostic Directive (IVDD), and whether the device was manufactured and labeled before the compliance start date (i.e., “existing devices”).
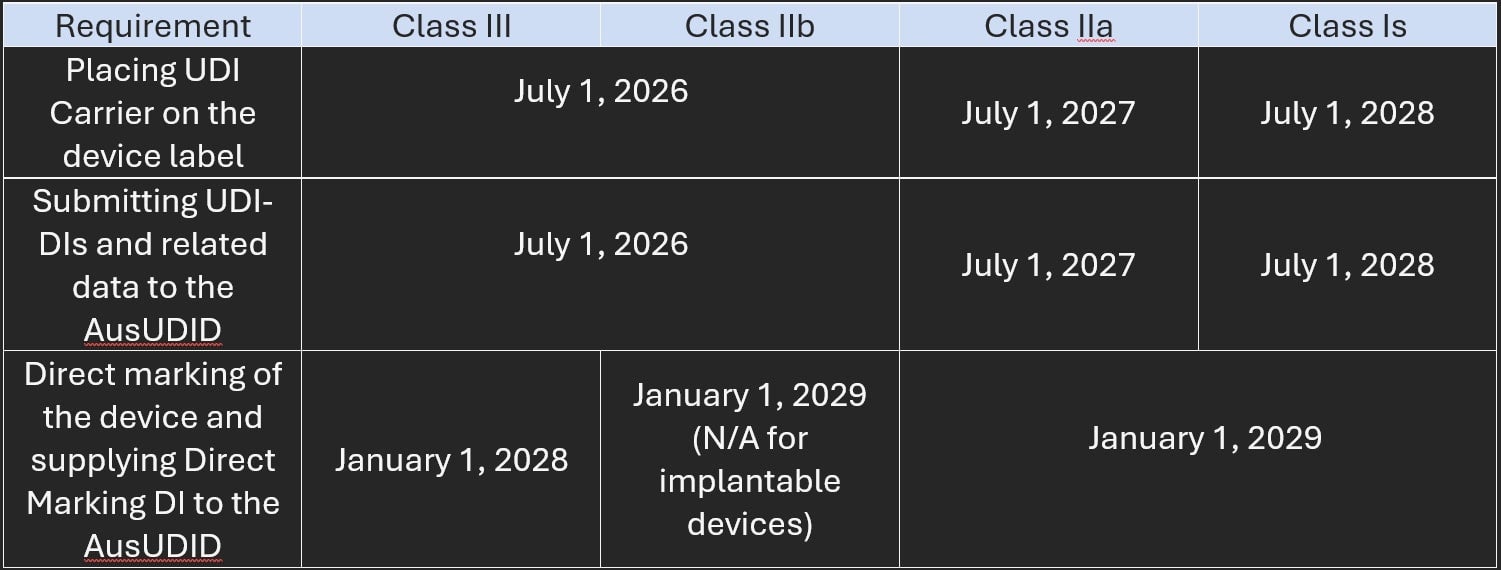
The TGA published new guidance, Complying with the Unique Device Identification Timeframes for Medical Devices, to elaborate on the dates, as follows:
Figure 1.

Figure 2.
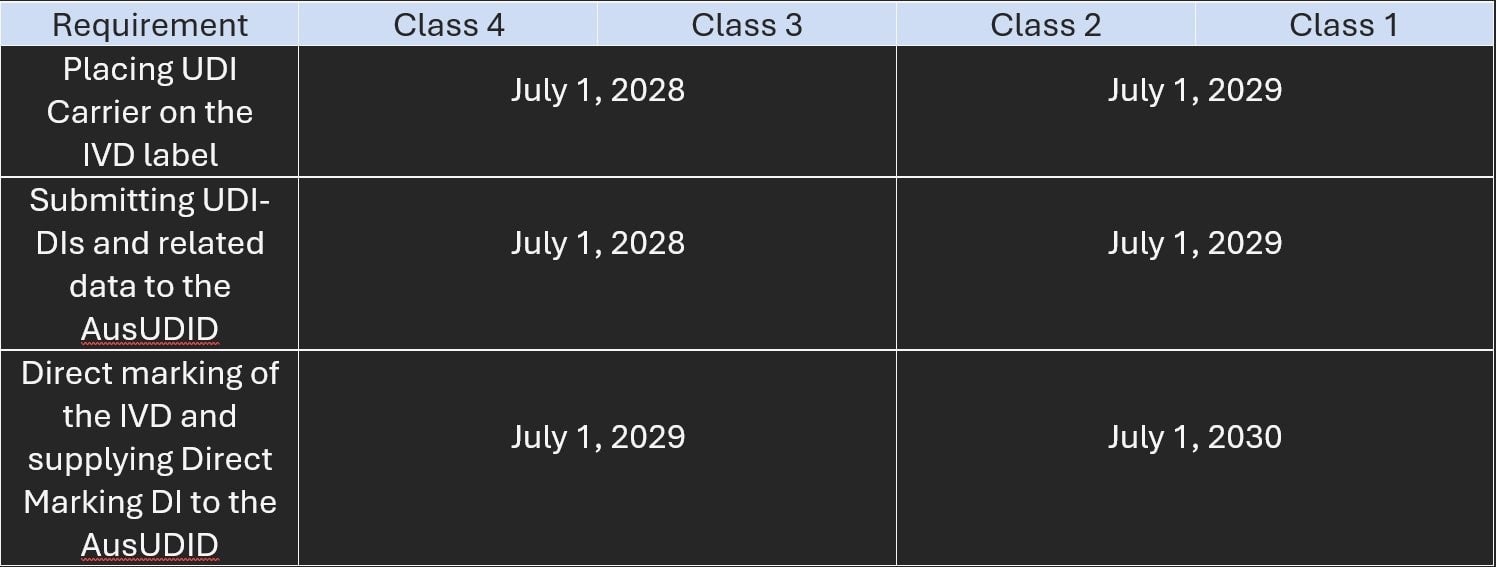
“Existing devices” classified as Class III or IIb must comply with the UDI requirements by the respective compliance start dates. “Existing devices” of all other classes are exempt for the lifetime of the device.
The Australian Unique Device Identification Database
The TGA has established and released the AusUDID to store UDIs and related data for devices supplied in Australia and this data links to the relevant entry in the Australian Register of Therapeutic Goods (ARTG).
The AusUDID contains two unique environments: the “production” environment contains current medical device information that can be freely accessed by the public. The “pre-production” environment is only available to sponsors and manufacturers and is intended to help sponsors and manufacturers familiarize themselves with the AusUDID and test their respective data submission methods before submitting live data.
Concluding remarks
After years of deliberation and development, the Australian UDI regulatory framework has arrived, bringing with it enhanced identification and traceability of devices to support a more effective regulatory framework.
Sponsors and manufacturers may voluntarily comply with the UDI requirements in advance of the deadlines, though they should then comply with all provisions of the UDI requirements to minimize end-user confusion.
Request more information from our specialists
Thanks for your interest in our products and services. Let's collect some information so we can connect you with the right person.






